Years for FDA to evaluate everything: 1,369,863,014
Years for decentralized system: 273.97🔬 Decentralized FDA
The Solution: Just Let People Try Stuff (Carefully)
Here’s a thought that apparently never occurred to anyone in Washington:
What if sick people could just… try treatments?
And then we could… write down what happens?
And then other sick people could… see what worked?
I know, I know. Without 17 years of paperwork, how would we know if medicines are safe? We’d have to rely on primitive methods like “observing if people get better or die.” It’s a bold strategy, considering our current one leaves 95% of rare diseases with zero approved treatments and ensures 85% of patients are barred from the trials that might save them. We’ve managed to perfect a system where only the healthy and compliant are allowed to test cures for the sick and desperate. It’s genius, in a suicidal sort of way.
This is because trials are designed to test drugs on people who don’t actually exist. To get into a study for an antidepressant, for example, you can’t have any other pesky problems like anxiety or PTSD. You can’t have a history of drug or alcohol use. You can’t be on other medications. You have to be the perfect kind of sick. The result? One investigation found that only 14.5% of real-world patients with depression would actually qualify. The rest of us are too messy for their beautiful, clean data.
On top of excluding everyone with a pulse, these “definitive” studies are run on comically small groups. They’ll test a new heart drug on 275 people, a cancer drug on just 20 people, and a diabetes drug on 100 people, then prescribe the winner to millions.
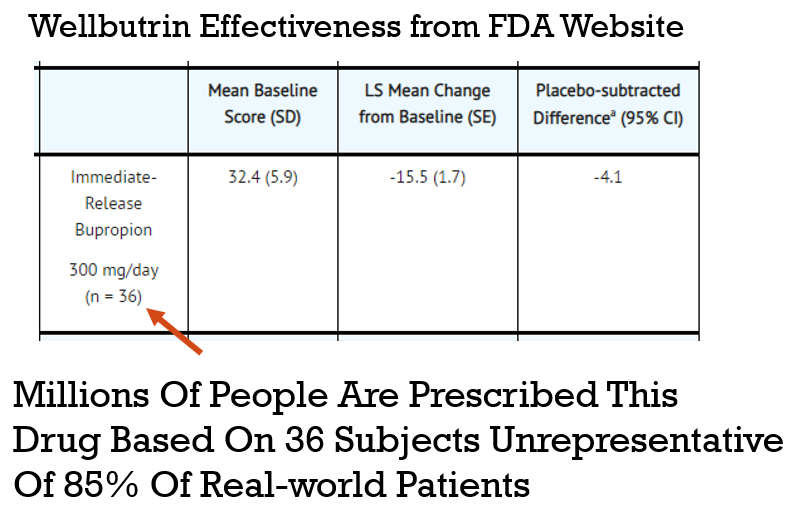
We’re basing the survival of our species on a sample size smaller than a kindergarten class. So, instead of studying these mythical, perfectly sick unicorns, what if we just collected data from everyone who’s actually sick?
The Power of Real-World Evidence
The dFDA is built on a simple but powerful idea: we can learn more, faster, by analyzing data from real patients in the real world. This is often called Real-World Evidence (RWE).
The Potential of RWE-Based Studies
- Diagnostics - Data mining and analysis to identify causes of illness
- Preventative medicine - Predictive analytics and data analysis of genetic, lifestyle, and social circumstances to prevent disease
- Precision medicine - Leveraging aggregate data to drive hyper-personalized care
- Medical research - Data-driven medical and pharmacological research to cure disease and discover new treatments and medicines
- Reduction of adverse medication events - Harnessing of big data to spot medication errors and flag potential adverse reactions
- Cost reduction - Identification of value that drives better patient outcomes for long-term savings
- Population health - Monitor big data to identify disease trends and health strategies based on demographics, geography, and socioeconomic
Cost Savings in Drug Development
Failed drug applications are expensive. A global database of treatments and outcomes could provide information that could avoid massive waste on failed trials.
- A 10% improvement in predicting failure before clinical trials could save $100 million in development costs.
- Shifting 5% of clinical failures from Phase III to Phase I reduces out-of-pocket costs by $15 to $20 million.
- Shifting failures from Phase II to Phase I would reduce out-of-pocket costs by $12 to $21 million.
Cost Savings Through Decentralization
But Isn’t This Just “Observational Research”?
When people hear “real-world evidence,” they often think of flawed observational studies from the past.
Why It Seems Like Diet Advice Flip-Flops All the Time
In 1977, the USDA and Time Magazine warned Americans against the perils of dietary cholesterol. Yet, in 1999, TIME released a very different cover, suggesting that dietary cholesterol is fine.

The problem with these old studies is that correlation is not the same as causation.

A common source of error is the “healthy person bias.” For instance, if an observational study finds “People Who Brush Teeth Less Frequently Are at Higher Risk for Heart Disease,” it might be that people who brush their teeth are also more likely to exercise and eat well.
However, the massive amount of automatically collected, high-frequency longitudinal data we have today makes it possible to overcome these flaws.
Overcoming the “No Control Group” Problem
The primary flaw with old observational research is the lack of a control group. With modern data, a single person can act as their own control group using an A/B experimental design.
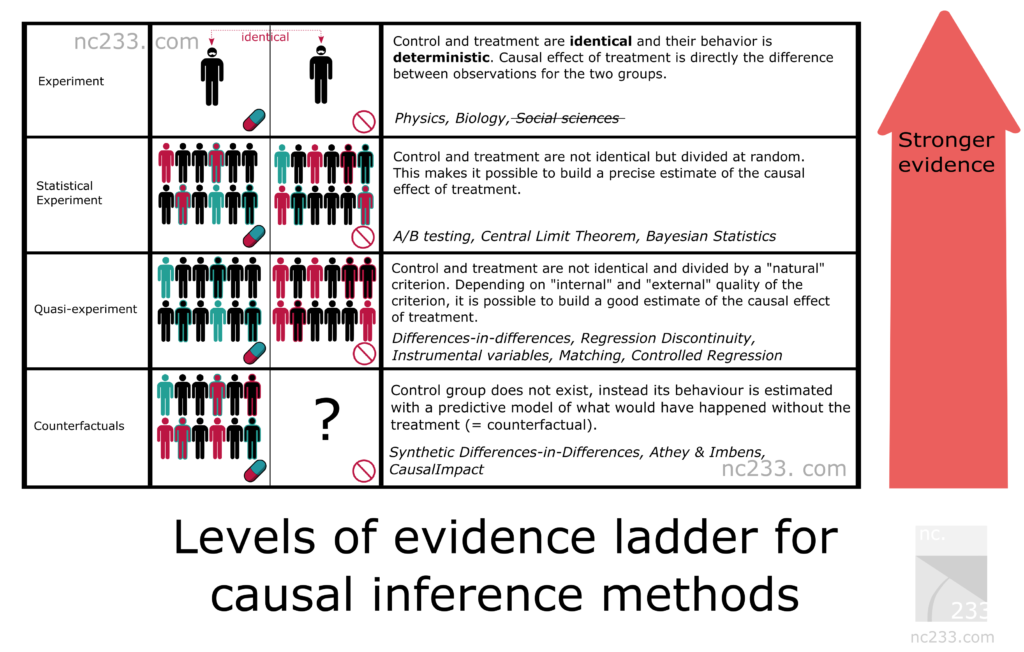
For instance, if someone with arthritis wants to test a Turmeric supplement, their experiment could look like this:
- Month 1: Baseline (Control) - No Turmeric
- Month 2: Treatment (Experimental) - 2000mg Turmeric/day
- Month 3: Baseline (Control) - No Turmeric
- Month 4: Treatment (Experimental) - 2000mg Turmeric/day
By repeating this, we can gain statistical confidence. We can also use advanced statistical models (like diffusion-regression state-space models) to create a “synthetic control group,” which predicts what would have happened without the intervention. This allows us to account for other variables like diet, exercise, and seasonality.
We can then apply Hill’s criteria for causation to quantify the likelihood of a causal relationship.
Meta-Analyses Support Real-World Evidence
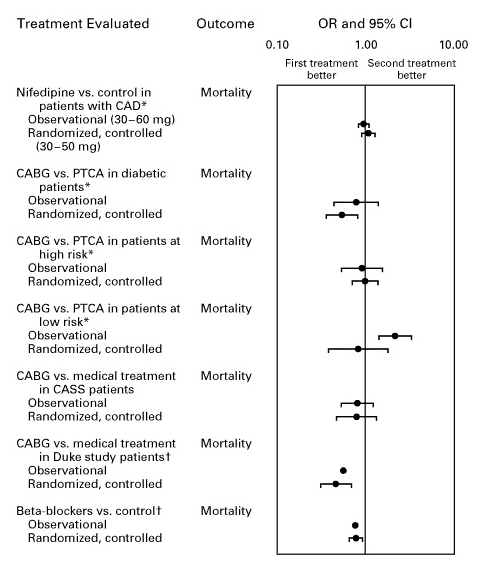
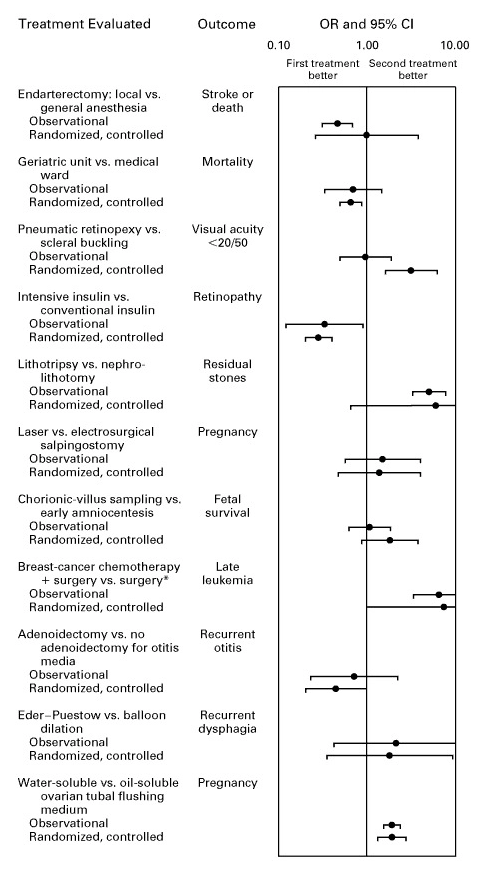
Modern statistical methods make a difference. A meta-analysis in the New England Journal of Medicine found that:
when applying modern statistical methodologies to observational studies, the results are generally not quantitatively or qualitatively different from those obtained in randomized, controlled trials.
Other meta-analyses confirm this, showing strong agreement between observational studies and RCTs for both mortality and various other outcomes.
Introducing FDA.gov 2.0: Now With 80% Less Death
The dFDA upgrades FDA.gov from a digital cemetery to something useful. Cost: Less than one fighter jet that doesn’t work.
Here’s the revolutionary process:
Step 1: Type in What’s Killing You

Revolutionary feature: A search box. The FDA’s current website doesn’t have this because they assume you’ve already died.
Step 2: See What Actually Works (Based on Reality, Not Theory)
Instead of “This drug is approved for exactly this condition in exactly these patients on exactly Tuesdays,” you get:
“Here’s what happened to 50,000 people who tried this:
- 40% got much better
- 30% got somewhat better
- 20% no change
- 10% grew a third nipple (but a useful one)”
It’s like Consumer Reports, but for not dying.
Treatment Rankings: Every Option, Ranked by Reality
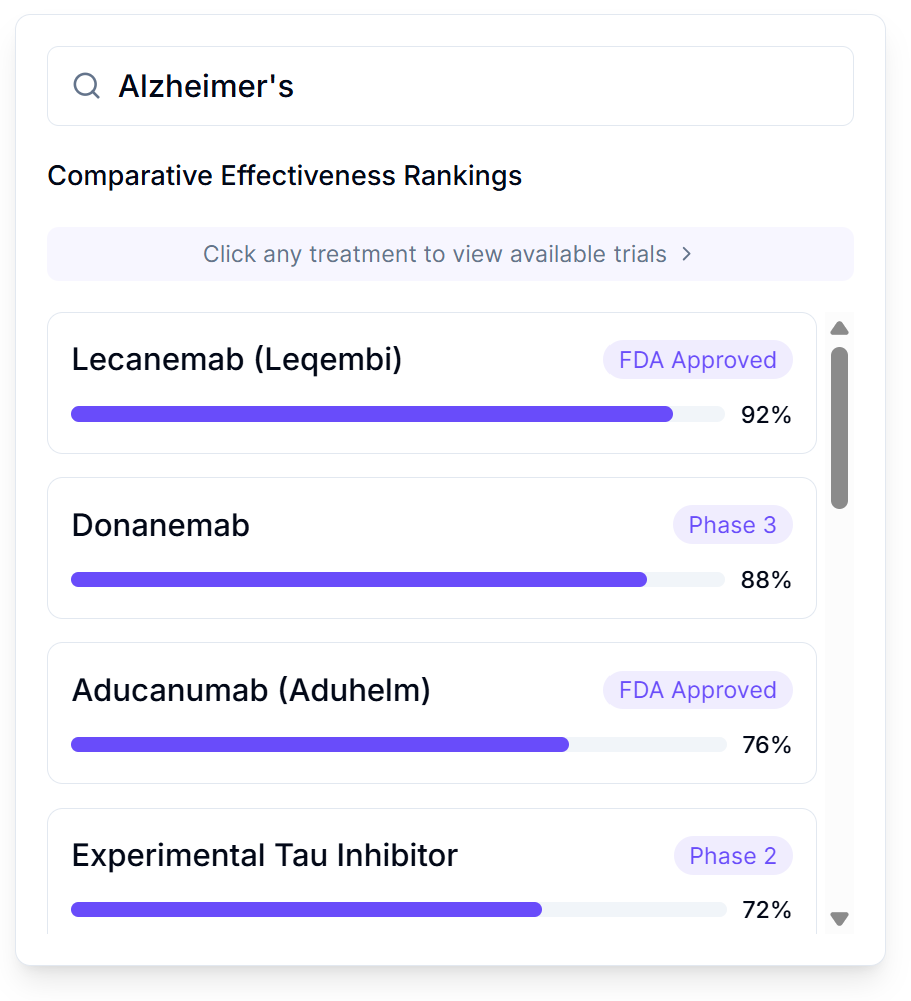
Search any condition and see every treatment ranked by real-world effectiveness:
- FDA Approved treatments with effectiveness scores from actual patients
- Phase 3 and Phase 2 trials you can join right now
- Experimental options with preliminary data
- One-click access to join available trials
No more guessing which treatment your doctor half-remembers from a conference. Just clear rankings based on what actually worked for people like you. The current system never publishes negative results, so we waste billions repeating the same failed ideas. This ends that particular brand of expensive stupidity.
Step 2.5: See the Future (With Financially-Backed Predictions)
To complement the real-world data from past patients, the dFDA will integrate a powerful forecasting tool: live prediction markets.
This isn’t just polling experts; it’s a marketplace where researchers, doctors, investors, and even the public can bet real money on a trial’s future outcomes.
For any given trial, you’ll see real-time odds on questions like:
- Effectiveness: “What are the chances this treatment will reduce tumor size by >50% in the next 6 months?” Market Price: 38%
- Adverse Events: “What is the probability of patients experiencing severe nausea?” Market Price: 22%
- Completion: “What are the odds this trial will be fully enrolled by its target date?” Market Price: 71%
Why Trust This?
- Skin in the Game: These aren’t cheap-talk opinions. Bettors lose money if they’re wrong, creating a powerful incentive for accuracy.
- The Ultimate Fact-Checker: The DIH automatically resolves these bets using the actual, aggregated, and anonymized patient data collected by the dFDA. If the market predicts a 22% rate of nausea, and the real-world data shows 40%, the market learns and adjusts—or bettors go broke.
This gives patients an invaluable second layer of information: not just what has happened, but what the world’s collective, financially-incentivized intelligence thinks will happen.
Step 3: Join a Trial from Your Couch (While Dying Comfortably)
Current system: Drive 500 miles to a university hospital, wait 6 months, get rejected for having the wrong kind of dying.
New system: Click button. Get pills. Report if you die.
Revolutionary!
Step 4: Get Drugs Delivered Like Pizza (But More Life-Saving)
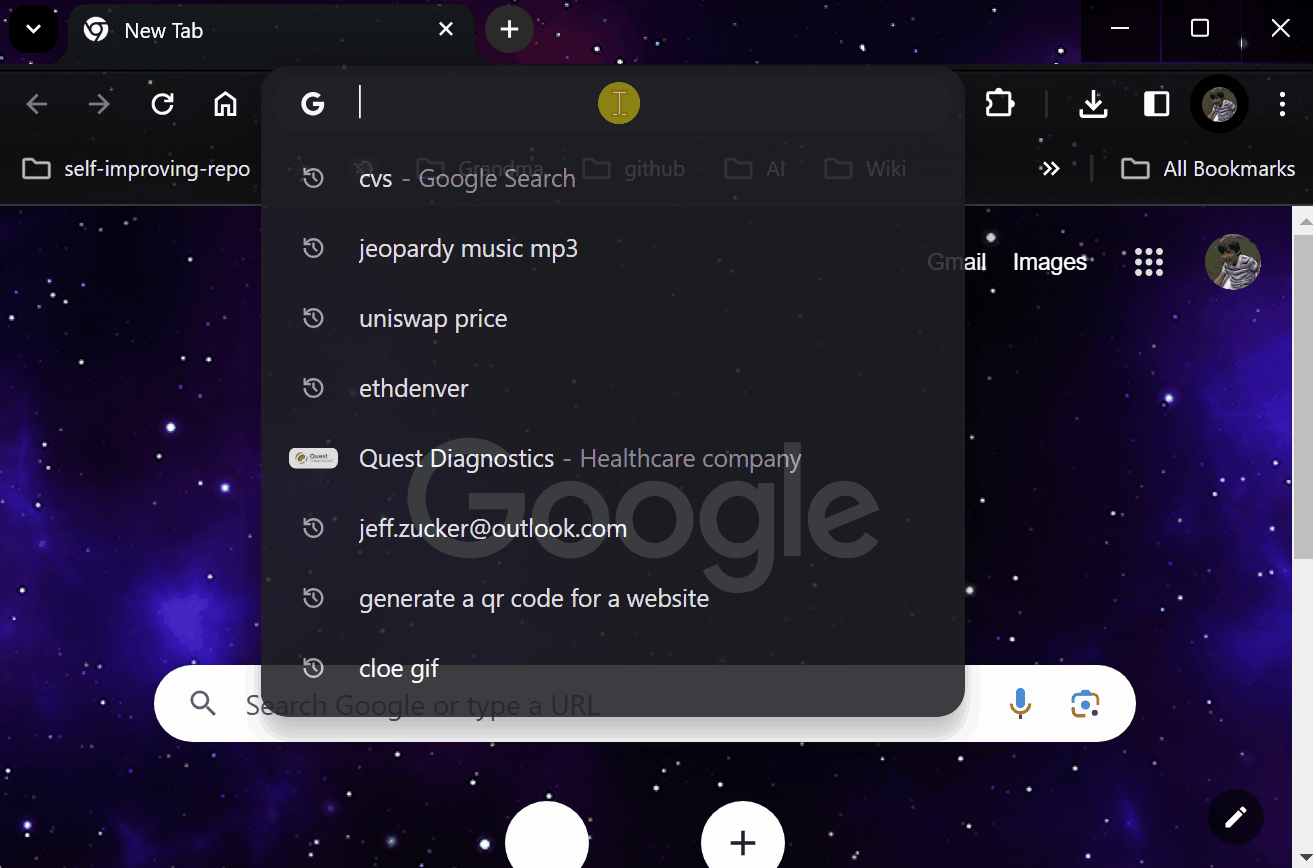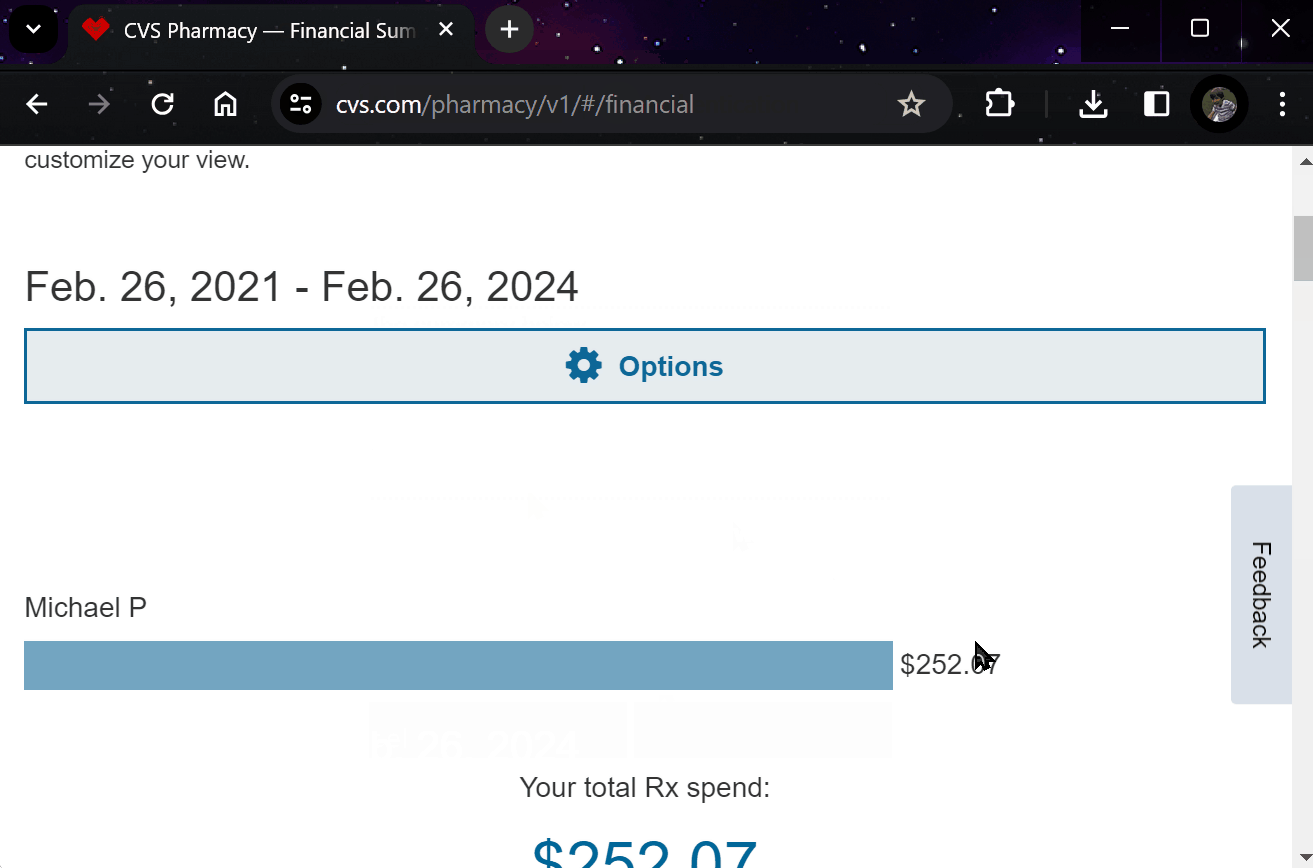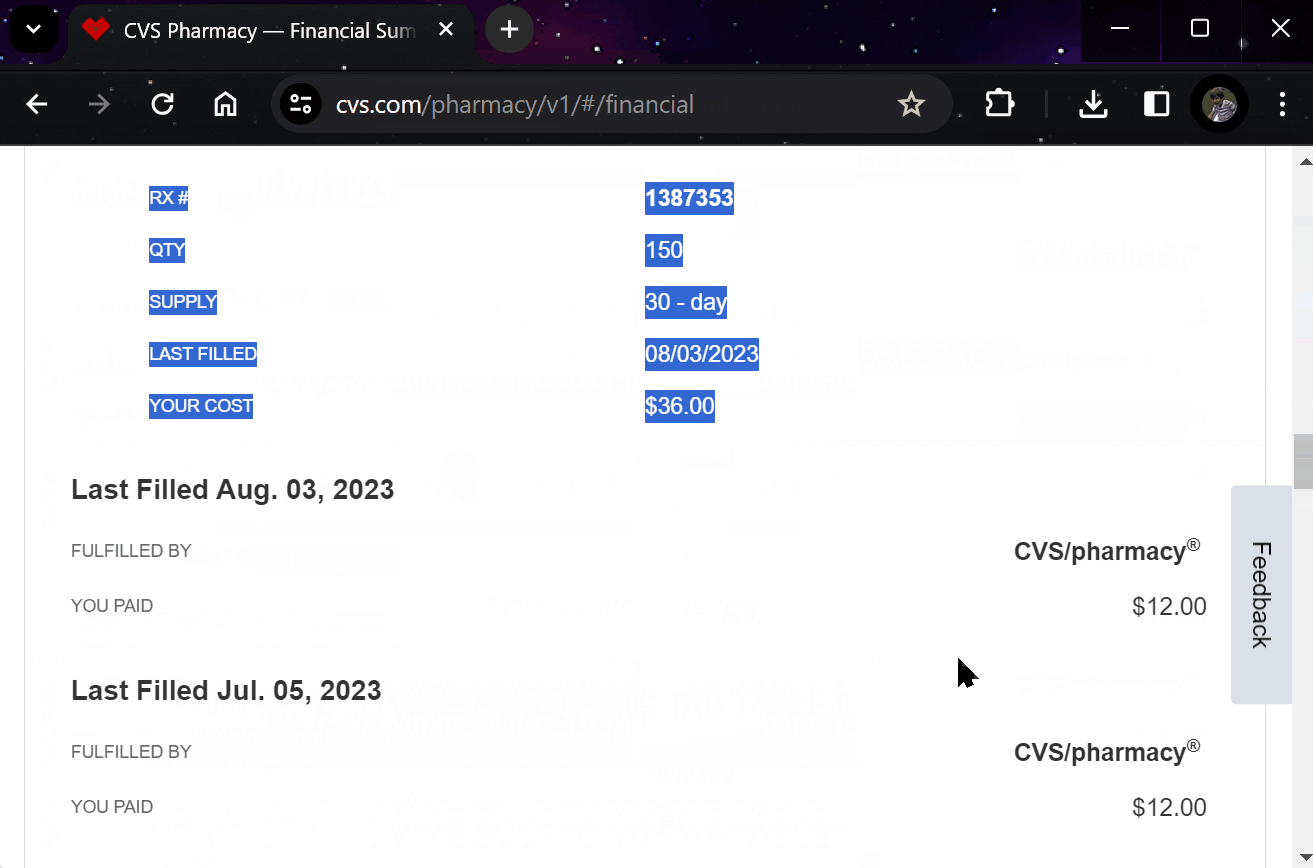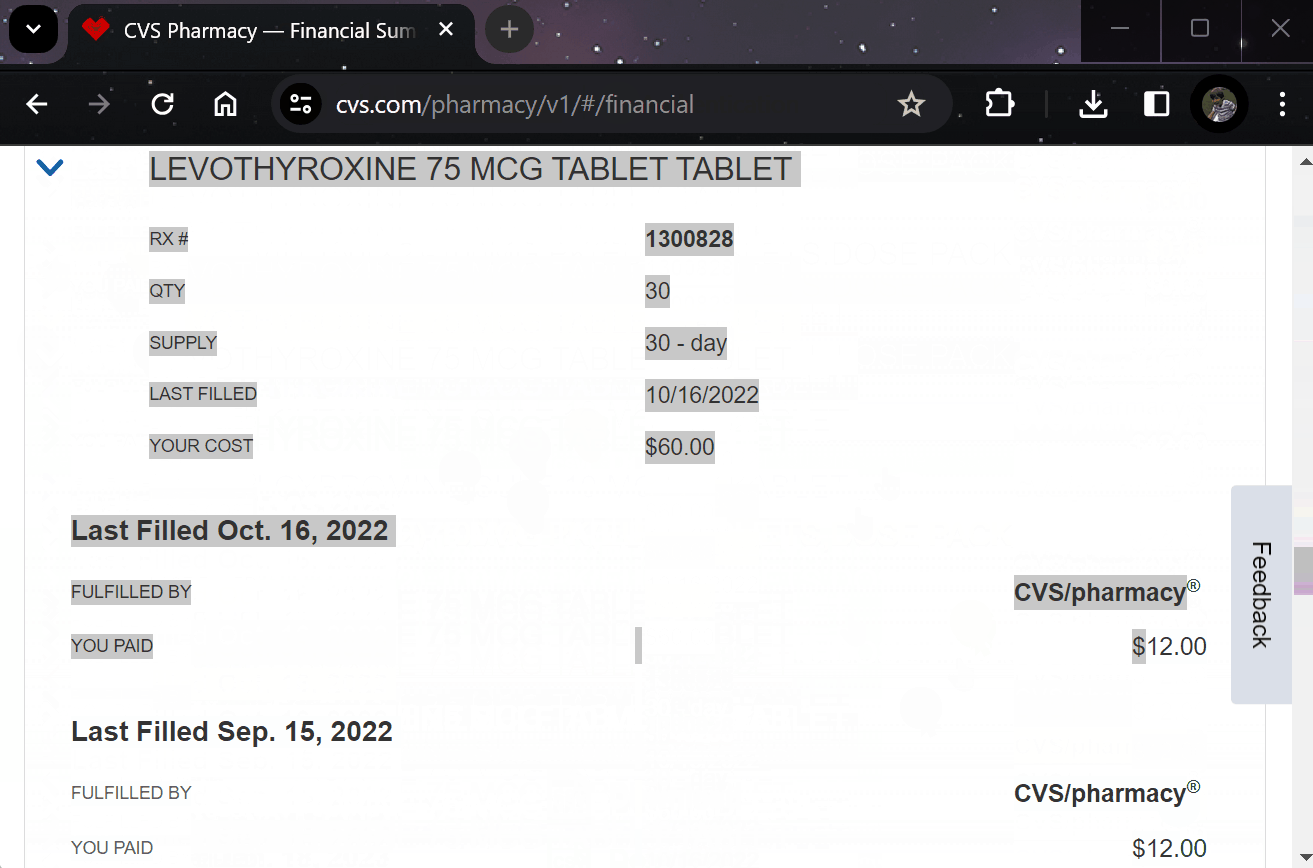
Amazon can deliver a banana costume in 2 hours but experimental medications take 6 months and require seven forms of ID?
The dFDA fixes that. Your pharmacy becomes a trial site. Your doctor becomes a researcher. Your dying becomes data.
Step 5: Report Results Without a PhD in Paperwork
Current system: 500-page case report forms that ask questions like “Rate your suffering on a scale of mauve to burnt sienna.”
New system:
- “Are you dead?” Yes/No
- “If no, how dead do you feel?” Slider bar
- “Any new body parts?” Check all that apply
Step 6: Everyone Benefits from Everyone’s Suffering
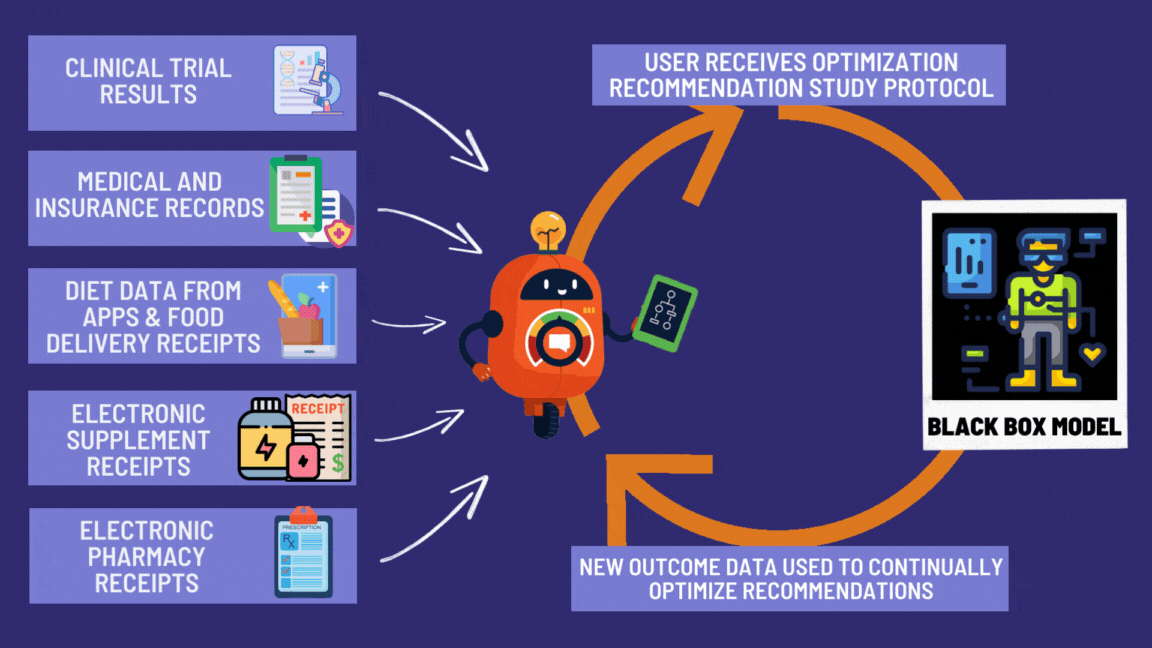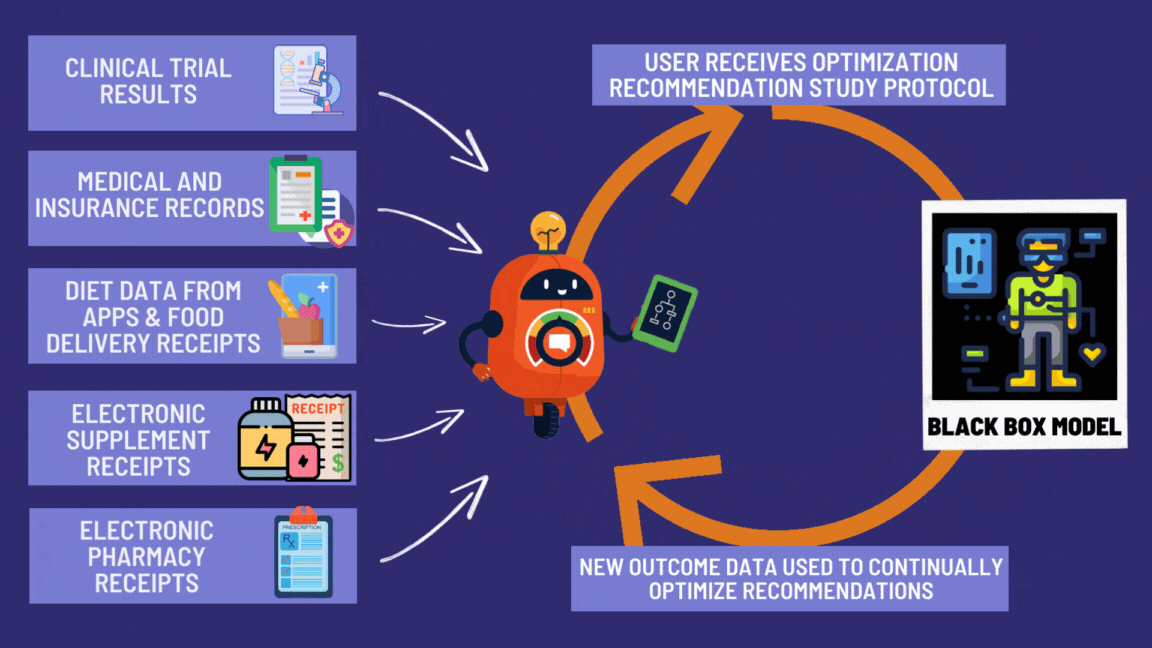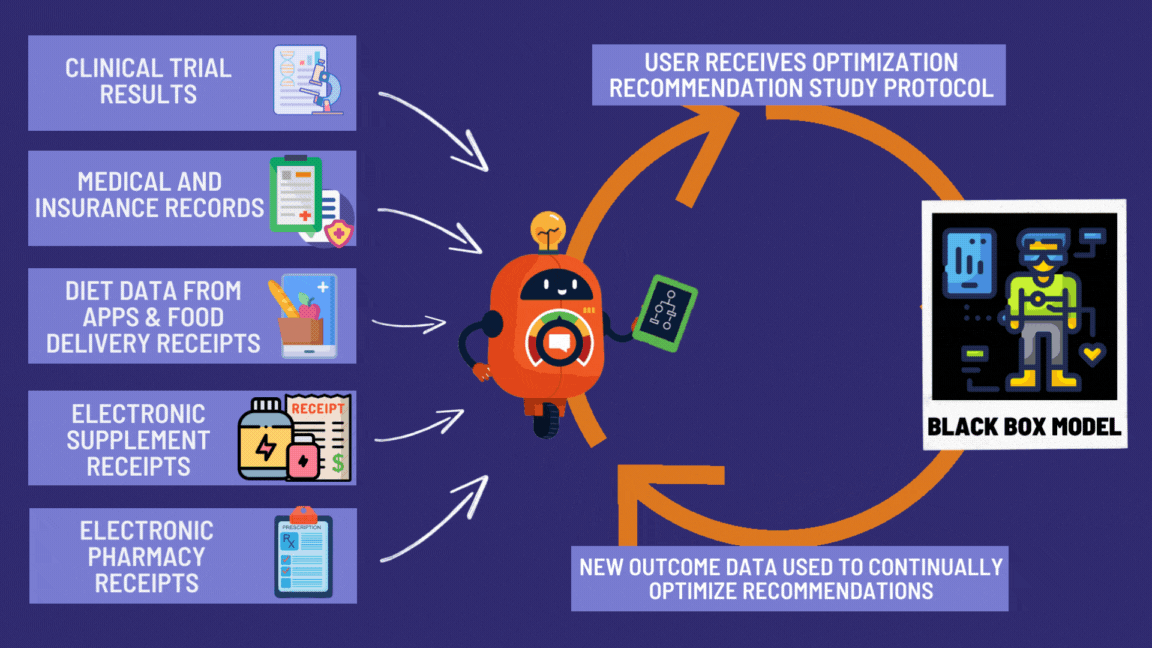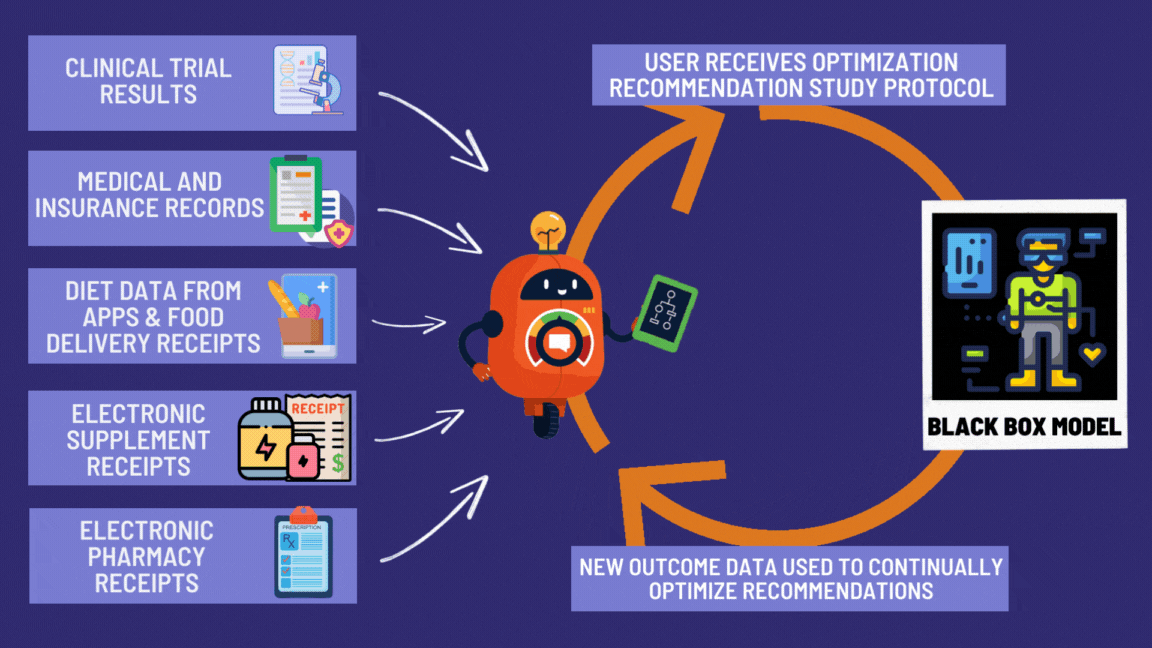
Your data helps the next person. Their data helps you. It’s socialism, but for not dying.
Every pill becomes a tiny experiment. Every patient becomes a scientist. Every outcome gets recorded.
It’s what we should have been doing since we invented writing.
The Money Shot: How We Save 95% on Not Killing People
Look at these beautiful savings! It’s like Black Friday, but for survival!
| What We’re Fixing | Current Insanity | New Reality | Money Saved | Lives Saved |
|---|---|---|---|---|
| Clinical Phase Timeline | 9.1 years (avg) | 2-3 years | ~7 years of waiting eliminated | ~50,000/year |
| Cost per trial | $57 million | $2 million | $55 million | Infinite ROI |
| Who can participate | 15% of patients | 100% of patients | 85% inclusion rate | Everyone |
| Rare diseases with treatments | 5% | Eventually 100% | Priceless | Millions |
The Itemized Receipt of Stupidity We’re Eliminating
| Stupid Expense | What FDA Pays | What We’ll Pay | Savings | What It Actually Is |
|---|---|---|---|---|
| 💾 Data Management | $198,014 | $10,000 | 94.9% | Excel spreadsheets with fear |
| ✅ IRB Approval | $324,081 | $5,000 | 98.5% | Permission slips for adults |
| 📝 IRB Amendments | $6,347 | $0 | 100% | Permission to fix typos |
| 🔍 Source Data Verification | $1,486,250 | $25,000 | 98.3% | Checking if you lied about dying |
| 🤝 Patient Recruitment | $805,785 | $15,000 | 98.1% | Facebook ads for dying people |
| 🎯 Patient Retention | $76,879 | $20,000 | 74% | Bribes to keep dying people interested |
| 👨⚕️ Research Associates | $2,379,605 | $150,000 | 93.7% | People who watch you take pills |
| 👩⚕️ Physicians | $1,966,621 | $100,000 | 94.9% | Doctors pretending to be scientists |
| 🏥 Clinical Procedures | $5,937,819 | $1,000,000 | 83.2% | Poking you with expensive things |
| 🧪 Laboratory | $2,325,922 | $500,000 | 78.5% | Testing if your pee is still pee |
| 🏢 Site Recruitment | $849,158 | $0 | 100% | Bribing hospitals to participate |
| 🏗️ Site Retention | $4,461,322 | $0 | 100% | Bribing hospitals to not quit |
| 👥 Administrative Staff | $7,229,968 | $100,000 | 98.6% | People who file the files |
| 📊 Site Monitoring | $4,456,717 | $0 | 100% | People who watch the people who watch you |
| 🏢 Site Overhead | $7,386,816 | $0 | 100% | Electricity for the filing cabinets |
| 📎 All Other | $17,096,703 | $100,000 | 99.4% | Nobody knows but it’s expensive |
| TOTAL STUPIDITY | $56,988,007 | $2,025,000 | 95.7% | The price of bureaucracy |
This eliminates $55 million of paperwork per trial. That’s 55 million pieces of paper nobody reads, filed in cabinets nobody opens, in buildings nobody visits.
Those papers killed more people than they saved.
Why This Works: The Mathematical Impossibility of Committees
Here’s the problem with having ~200 FDA bureaucrats decide what 8 billion people can try when they’re dying:
The Math That Proves Centralization Can’t Work
Let’s calculate how long it would take the FDA to properly evaluate every treatment for every person:
The committee needs 1.3 million years. The decentralized system needs 100 days.
Why 200 People Can’t Replace 8 Billion
The FDA doesn’t know:
- What disease you have (they haven’t met you)
- How your body responds to treatments (genetics are unique)
- What risks you’re willing to take (some prefer death to side effects, others the reverse)
- Whether you’d rather die trying or die waiting (only you can answer this)
But they decide for you anyway.
The Solution: Let 8 Billion Experts Make 8 Billion Decisions
Current system:
330 Million Americans
↓
200 FDA Bureaucrats (Bottleneck)
↓
17-Year Delays
↓
Millions DiedFDA system:
8 Billion Humans
↓ ↓ ↓ ↓ ↓ (Parallel Processing)
Millions of Trials Simultaneously
↓
Real-Time Results
↓
Data Saves EveryoneThis isn’t ideology. It’s information theory. You mathematically cannot centralize medical decisions for 8 billion unique people. The FDA could work 24/7 for a million years and still be behind.
The only solution is to let patients and doctors make their own decisions, then pool the data so everyone benefits from everyone’s experience.
Real Examples of This Working (While the FDA Wasn’t Looking)
The Oxford Recovery Trial: How the British Accidentally Saved Medicine
During COVID, while America was filling out forms, Oxford University did something crazy: they just tested drugs on dying people to see if they stopped dying.
Cost per patient: - Normal clinical trials: $41,000 - Oxford pragmatic trials: $500
That’s not a typo. Five hundred dollars. The cost of a nice dinner in Manhattan to save a human life.
Results:
- Found that steroids cut COVID deaths by 30%
- Saved over 1 million lives globally
- Took 3 months instead of 3 years
- Cost less than one Super Bowl commercial
The FDA’s response: “But did they file the correct paperwork?”
Wikipedia for Whether You’ll Die
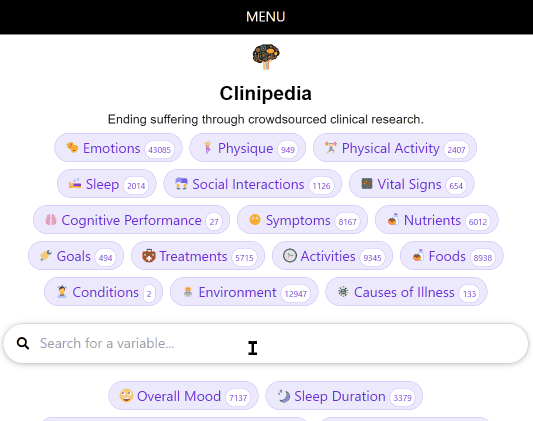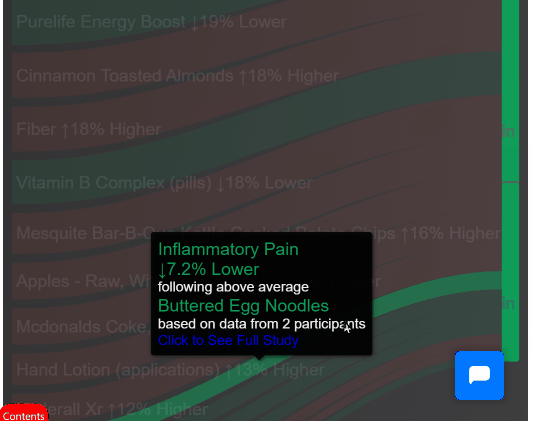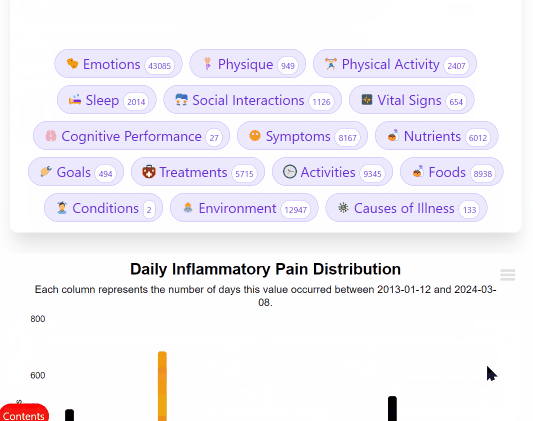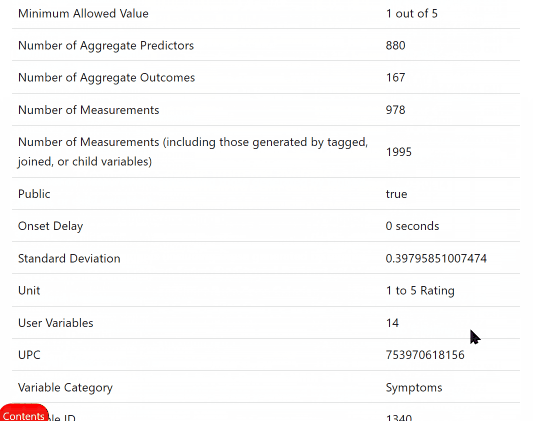
The system includes Clinipedia: Wikipedia but instead of arguing about whether tomatoes are fruits, we argue about whether they cure cancer.
Every treatment gets a page. Every outcome gets recorded. Every patient contributes data.
Imagine looking up your disease and seeing:
“Exploding Kidney Syndrome”
From Clinipedia, the encyclopedia of not dying
Current Treatments Ranked by Not-Dying Score:
- Prayer (3% effective, 0% side effects)
- Experimental Drug XJ-47 (67% effective, 30% chance of jazz hands)
- Traditional medicine (5% effective, tastes like sadness)
- Doing nothing (0% effective, 100% chance of exploded kidneys)
- Essential oils (0% effective, room smells nice while you die)
This page based on data from 10,847 patients who contributed their suffering to science
Become a Scientist from Your Couch
The system also lets you create your own studies. Got a theory that wearing socks inside-out cures baldness? Create a study. Get a link. Invite your balding friends to join. The system handles the rest. Your data gets published. Who knows, maybe you’ll win a Nobel Prize. Or maybe you’ll just prove that socks are socks. Either way, humanity learns something without spending two billion dollars.
Your Personal Death-Prevention Assistant: The FDAi
Everyone gets a superintelligent doctor that lives in their phone and doesn’t judge them for Googling “is my poop normal?”
The FDAi (Food and Drug Artificial intelligence) is like Siri, but instead of telling you the weather, it tells you how not to die.
You: “I have diabetes and my foot fell off.” FDAi: “Based on 50,000 similar cases, here’s what worked:
- 60% success: Reattachment surgery + Drug A
- 30% success: Prosthetic foot + Drug B
- 10% success: Hopping lessons + Prayer
- 0% success: Essential oils (but your remaining foot will smell lavender-fresh)”
It does this by connecting to all your data—from wearables, apps, and your medical records—and looking for patterns. It’s automated root cause analysis for your own body, finding what’s wrong while your doctor is still trying to remember your name. It’s like having every doctor who ever lived in your pocket, except they actually agree on something.
The Digital Twin Safe: Your Medical Data’s Panic Room

Right now, your medical data is scattered across:
- 17 different doctors who don’t talk
- 5 insurance companies that hate you
- 3 pharmacies that can’t spell your name
- 1 veterinarian (long story)
The Digital Twin Safe puts it all in one place. It’s like a Swiss bank account, but for your cholesterol levels.
You control who sees what:
- Insurance companies: “He’s mostly alive”
- Doctors: Everything
- Researchers: Anonymized data
- Facebook: Absolutely nothing, Mark
Outcome Labels: Nutrition Facts for Drugs
Food has nutrition labels. Cigarettes have warning labels. Drugs have… incomprehensible 40-page inserts written by lawyers having seizures.
Outcome Labels fix that - clear, data-driven summaries showing exactly what happens when real people try a treatment:
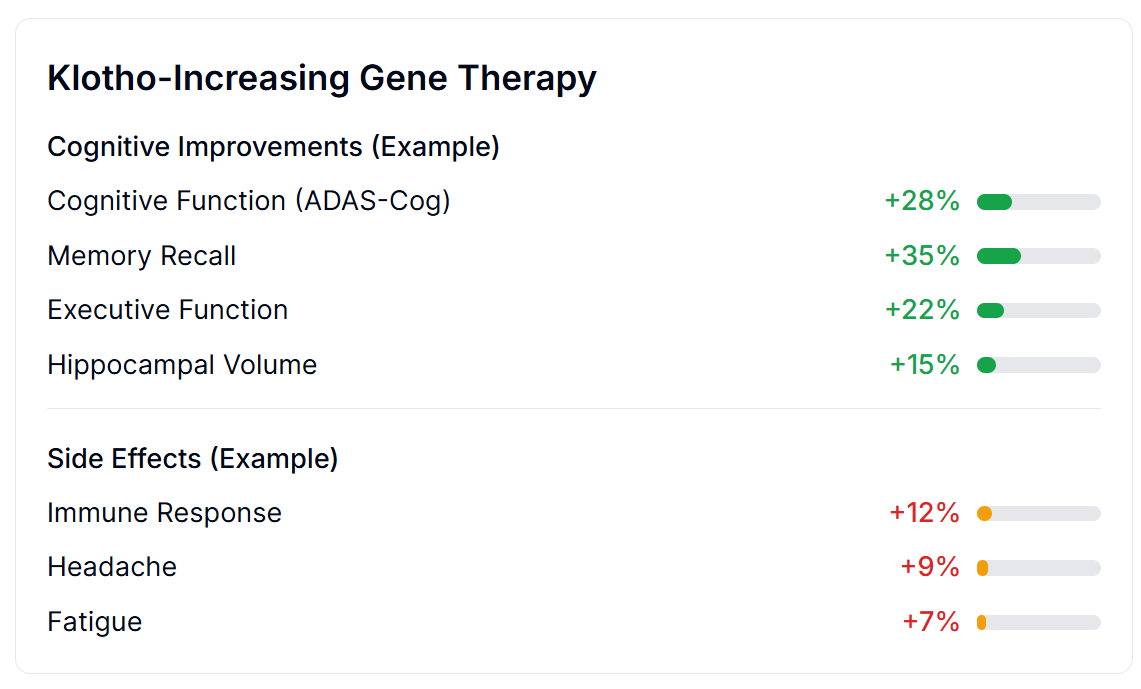
Based on thousands of real patients with real outcomes:
Cognitive Improvements:
- Memory Recall: +35%
- Cognitive Function: +28%
- Executive Function: +22%
- Hippocampal Volume: +15%
Side Effects:
- Immune Response: +12%
- Headache: +9%
- Fatigue: +7%
No marketing spin. No 40-page legal disclaimers. Just clear data about what actually happens to people like you.
Example: DEPRESSION CRUSHER 3000™
Outcome Label
Based on 50,000 humans who tried this:
- 📈 Depression: ⬇️ 60% reduction
- 😴 Sleep: ⬆️ 30% improvement
- 🍆 Sex drive: ⬇️ 90% reduction (sorry)
- 🎺 Spontaneous jazz hands: 15% chance
- 💀 Death: 0.01% (better than depression!)
This label based on actual humans, not laboratory rats who’ve never paid rent
The Disease Eradication Act: Making It Legal to Not Die
We need a law that says: “Dying people can try things that might help them not die.”
The fact that we need a law for this tells you everything about our species.
The Right to Trial Act (working title: “The Stop Being Stupid About Medicine Act”):
Section 1: If you’re dying, you can try experimental treatments. Section 2: We’ll write down what happens. Section 3: Other dying people can read what we wrote. Section 4: Repeat until nobody’s dying. Section 5: Seriously, how was this not already the law?
Congress will debate this for 17 years, the same time it takes to approve one drug.
The Future: Where Death Becomes Embarrassing
2027: The Beginning of the End of Dying Slowly
FDA.gov becomes actually useful. Millions join trials from home. The first “Wikipedia disease” gets cured entirely through crowdsourced trials. The FDA claims credit.
2030: Big Pharma Pivots or Dies
Pharmaceutical companies realize they can’t charge $10,000 for pills that cost $1 to make when everyone can see the data. Some adapt. Some become museums.
2035: The Great Revelation
We realize we could have done this all along. We had the internet since 1990. We had computers since 1950. We had writing since 3000 BC.
We spent 5,000 years letting people die while we filled out forms.
History books will call us “The Paperwork Age” and children will laugh at our stupidity.
2050: Death Becomes Opt-In
Diseases are mostly solved. Death becomes a choice, like smoking or voting Republican. The FDA’s job becomes preventing people from becoming immortal too quickly (traffic is bad enough).
The last FDA form is filled out. It’s immediately lost. Nobody notices.
How the dFDA Actually Works
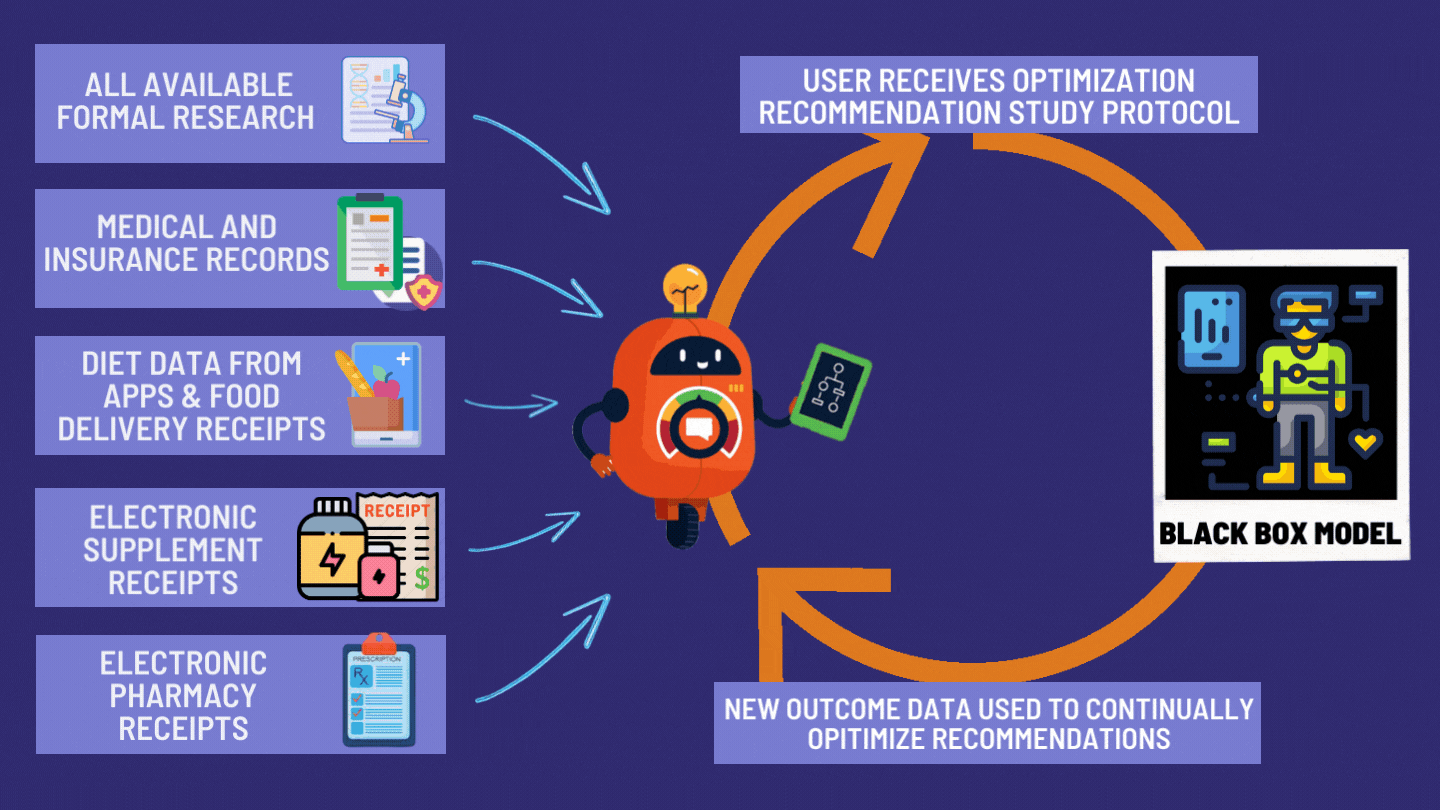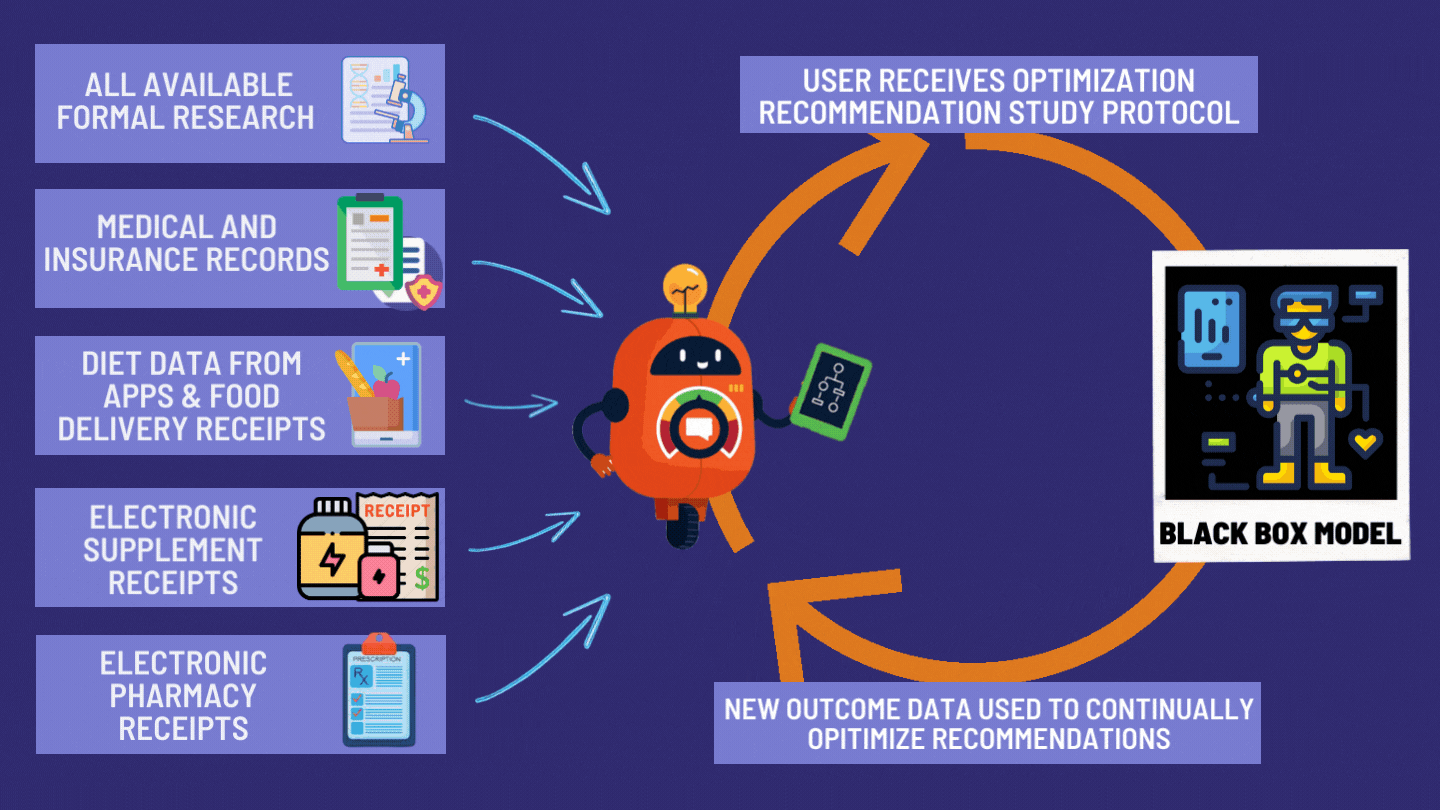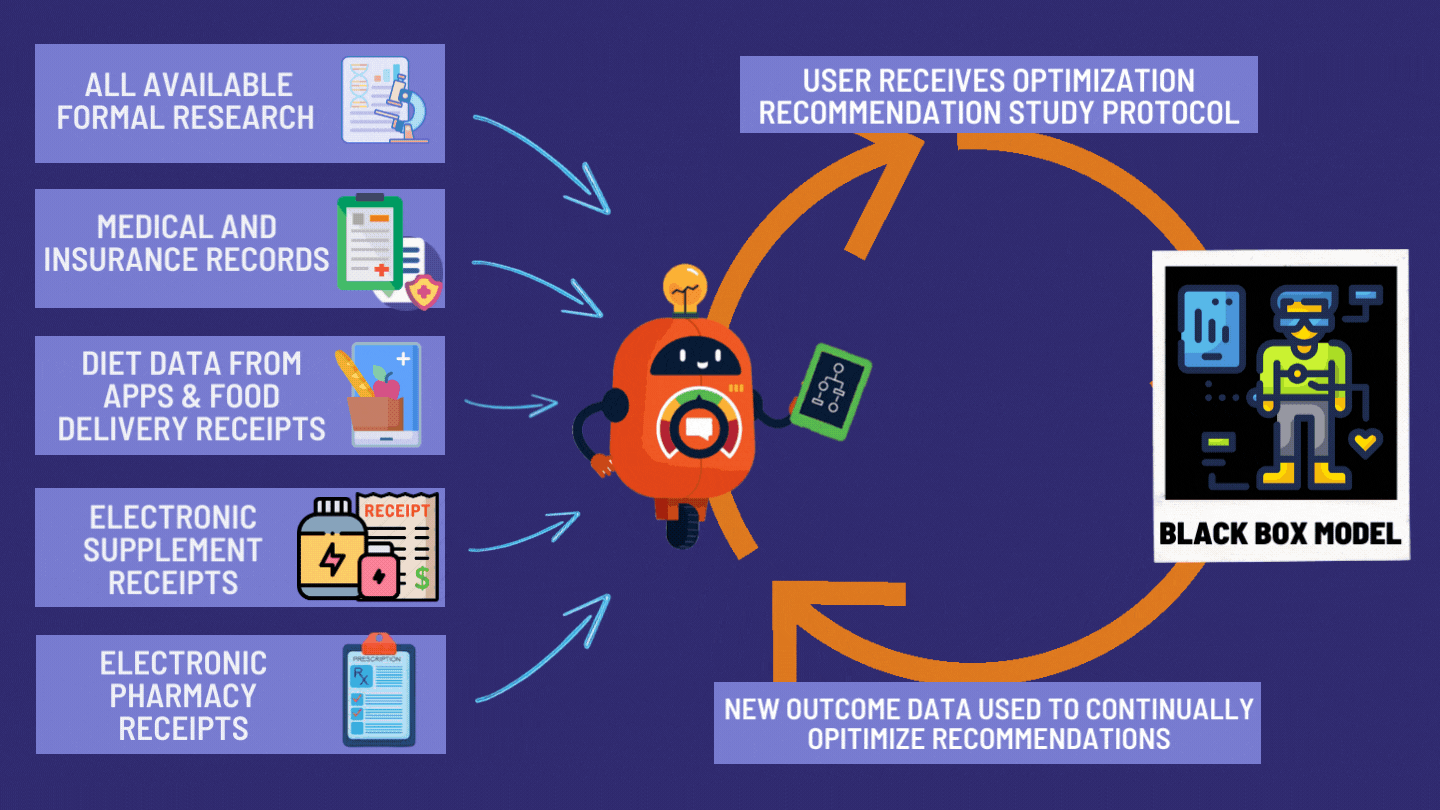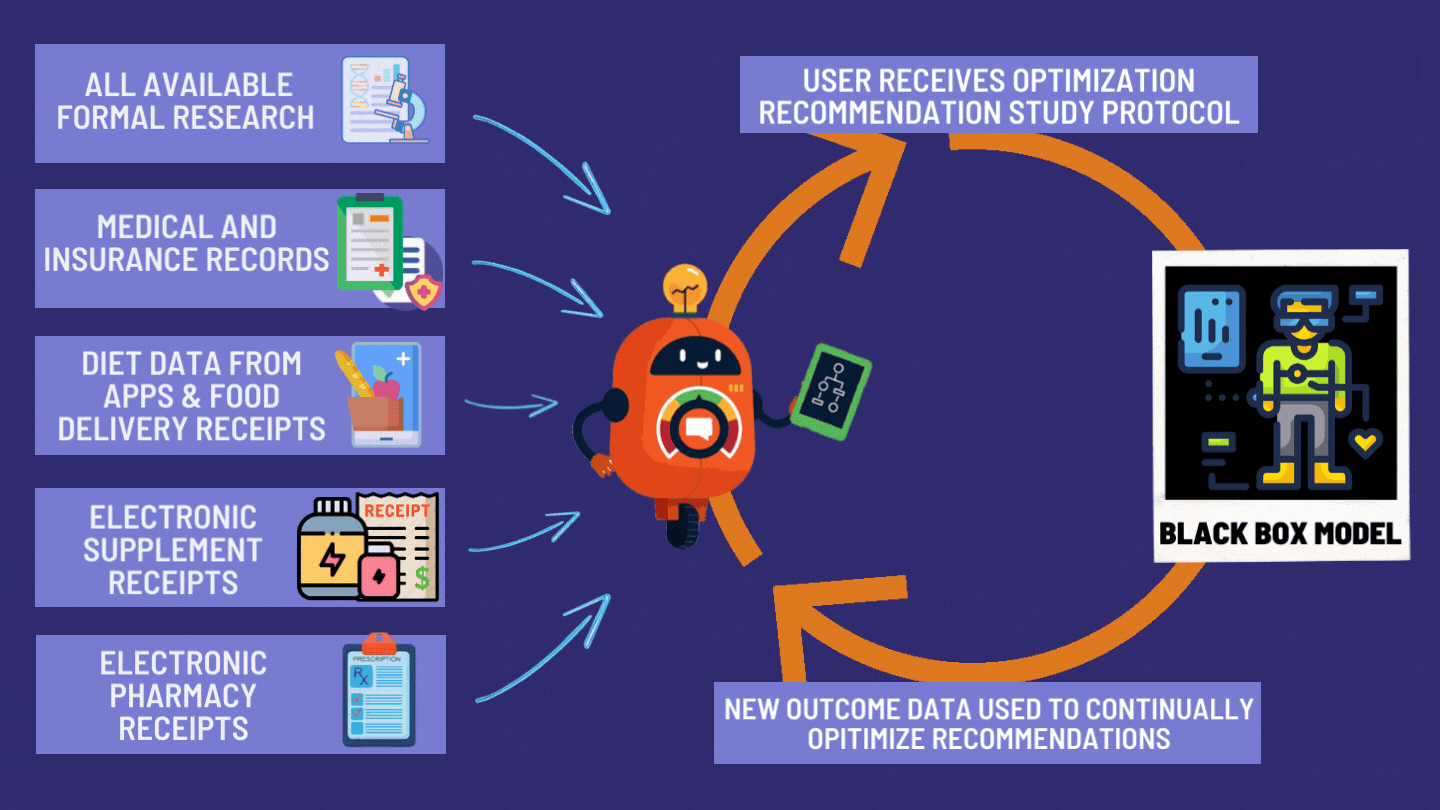
The dFDA achieves 82X More Efficiency through:
- Cost per patient: $500 vs the current $41,000
- Time to results: 2 years vs the current 17 years
- Patient access: Universal access vs 85% excluded currently
| Metric | Current FDA System | Decentralized FDA | Improvement |
|---|---|---|---|
| Approach | 📋 Central Planning | 🛒 Open Platform | Market-based |
| Cost per Patient | $41,000 | $500 | 82X cheaper |
| Time to Results | 17 years | 2 years | 8.5X faster |
| Patient Access | 15% | 100% | 6.7X more access |
| Innovation Impact | 70% approval drop after 1962 | Competition drives innovation | Reversed decline |